
Ben
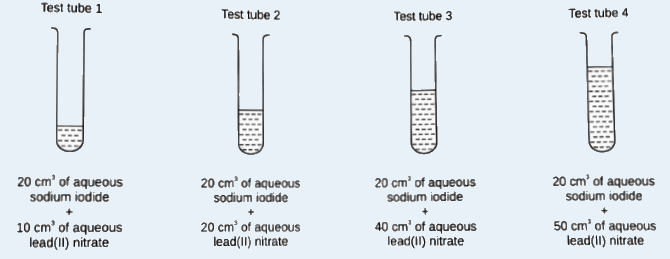
- hello, i dont understand why the answer is C. However, i know that in test tube 1, NaI is limiting, in test tube 2 both reagents are just nice, while in test tubes 3 and 4 Pb(NO3)2 is in excess. however, how does that affect amount of ppt?
 Hello!
Hello!- hi
 Let me take a look at the question
Let me take a look at the question- np
 So you just need me to explain to you what’s happening in test tube 3 and 4?
So you just need me to explain to you what’s happening in test tube 3 and 4?- yeah like how does lead (II) nitrate being excess or limiting affect the amt of ppt formed
 Since lead nitrate is in excess, technically we do not need to consider it.
Since lead nitrate is in excess, technically we do not need to consider it.
We just need to look at the number of moles of sodium iodide.
One mole is sodium iodide produces one mole of lead iodide. So for test tube 3 and 4, the number of sodium iodide is the same. Thus, the amount of lead iodide produces is the same for both test tubes.
So for test tube 3 and 4, the number of sodium iodide is the same. Thus, the amount of lead iodide produces is the same for both test tubes. Can you understand the explanation? ?
Can you understand the explanation? ?- ohh ok so when lead nitrate goes from limiting to just nice ppt increases but when its in excess ppt remaisn the same?
 Yes, that’s right.
Yes, that’s right.
For any equations, you do not need to consider the reactant that is in excess. You only consider the limiting reactant and do the calculation from there.- alright got it. thanks!











 Adeline Lee,
Adeline Lee, 



